|
|
 |
|
|
|
 |
| |
|
|
 |
The Oceans
Basics |
The special properties of water
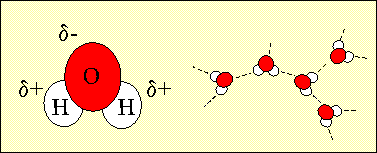
71% of the Earth is covered by water and 97% of this water is in the oceans. Water is made up of two atoms of hydrogen and one atom of oxygen. Because of water's electronic structure, the oxygen atom has a slight negative charge on it and the hydrogen atoms are slightly positive. When water molecules are close together, their positive and negative regions are attracted. These attractive forces are known as hydrogen bonds. Hydrogen bonds are the reason for water's very special properties which make life on Earth possible.
|
 |
 |
|
1. The structure of water showing the slight negative charge on the oxygen atom and the slight positive charges on the hydrogen atoms. The charged nature of the water molecule enables it to form hydrogen bonds with other water molecules. Author: Lucinda Spokes.
|
|
|
|
|
 |
- Water is the only natural substance that is found as a gas (water vapour), a liquid and a solid (ice) on Earth.
|
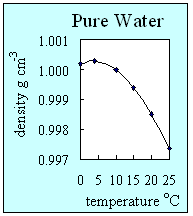
- Density is a measure of how compact a substance is. It is defined as the mass of a substance divided by its volume. Solids are almost always the most dense form of a substance, then liquids and then gases. As temperature increases, the density generally decreases. Pure water is an exception to this and is the only substance which has its highest density as a liquid. Water is at its most dense at about 4 oC. This is because hydrogen bonds between water molecules give ice a very stable open ordered structure. At low temperatures, water has a higher density than ice and this means that ice floats.
|
 |
 |
 |
|
2. How the density of pure water changes with temperature. This graph shows that pure water has its highest density at 4 oC when it is still a liquid. Author: Lucinda Spokes.
|
|
 |
 |
|
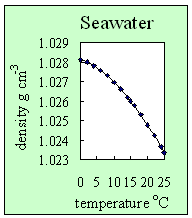
3. How seawater density changes with temperature. At a fixed salt concentration (in this case a salinity of 35), the density of seawater decreases with temperature. Author: Lucinda Spokes.
|
|
 |
- Adding salt to water increases its density. It also prevents the formation of hydrogen bonds. This means seawater, unlike pure water, doesn't have its maximum density at 4 oC, but when it freezes into ice. It also means that seawater freezes below 0 oC (this is why we put salt onto roads on cold nights to lower the risk of ice being formed).
|
- Water has a very high specific heat capacity. This means that a lot of energy is needed to increase its temperature (energy is needed to overcome the hydrogen bonds). As the Earth is 71% water, energy from the sun causes only small changes in the planet's temperature. This stops the Earth getting too hot or too cold and makes conditions possible for life. Heat is stored by the ocean in summer and released back to the atmosphere in winter. Oceans, therefore, moderate climate by reducing the temperature differences between seasons.
|
 |
 |
 |
|
4. It takes four times as much energy to heat water by 1 oC than it does to heat air. Author: Lucinda Spokes.
|
|
- Water also has a high heat of vaporisation. This means a lot of energy from the sun is needed to turn liquid water into vapour. As water vapour moves from warm areas to cooler regions it changes back to a liquid and may form rain. This releases heat which warms the air. The enormous amount of energy involved powers the storms and winds on Earth.
- Many substances dissolve in water and are stabilised by the hydrogen bonds. This allows the transport of oxygen, carbon dioxide, nutrients and waste materials in water and makes biological processes possible.
- Because oil molecules are large and not electrically charged, they can't be broken down into smaller charged molecules and be stabilised by water. This means that they do not dissolve in water.
|
 |
 |
|
5. Photograph of waves breaking over a research ship by Wendy Broadgate. Other images from NOAA.
|
|
About this page:
author: Lucinda Spokes - Environmental Sciences, University of East Anglia, Norwich - U.K.
1. sci. reviewer:
2. sci. reviewer:
edu. reviewer:
last updated: 2003-12-10
|
|
 |
|









