|
|
 |
|
|
|
 |
| |
|
|
 |
The Oceans
Basics |
How oceans take up carbon dioxide
The most important greenhouse gas, apart from water vapour, is carbon dioxide (CO2). Levels have changed over time both naturally and because of humans. Much of the carbon dioxide produced by humans does not stay in the atmosphere but is stored in the oceans or on land in plants and soils. By far the largest carbon store on Earth is in sediments, both on land and in the oceans, and it is held mainly as calcium carbonate (CaCO3). The second biggest store is the deep ocean where carbon occurs mostly as dissolved carbonate (CO32-) and hydrogen carbonate ions (HCO3-). We think that about a third of the carbon dioxide from fossil fuel burning is stored in the oceans and it enters by both physical and biological processes.
|
|
|
|
|
 |
Physical processes
Carbon dioxide dissolves more easily in cold water than in warm water. It also dissolves more easily in seawater compared to pure water because seawater naturally contains carbonate ions. |
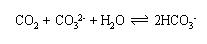 |
 |
|
|
|
Reaction of the carbon dioxide with carbonate produces hydrogen carbonate. Because of this reaction, only 0.5% of the inorganic carbon in seawater occurs as carbon dioxide gas. Since levels of carbon dioxide are so low in seawater, more carbon dioxide can enter the oceans from the atmosphere (the chemists will recognise this as an example of Le Chatelier's Principle). If the water stays at the surface and warms up as it moves around the globe, the carbon dioxide will relatively quickly escape back to the atmosphere. However, if the water sinks to the deep ocean, the carbon can be stored for more than 1000 years before ocean circulation returns it to the surface. Cold waters sink to the deep ocean at high latitudes in the Southern Ocean and in the Nordic and Labrador Seas in the North Atlantic Ocean. These regions are therefore the major physical carbon dioxide removal areas of the ocean.
|
Biological processes
As well as physical removal, carbon dioxide is also taken up by phytoplankton in photosynthesis and converted into plant material. Land plants and marine phytoplankton take up about the same amounts of carbon dioxide as each other but marine phytoplankton grow much much faster than land plants.
Most of the carbon dioxide taken up by phytoplankton is returned to the atmosphere when the phytoplankton die or are eaten but some is lost to the deep sea sediments in sinking particles. The sinking of this plant material is known as the biological pump because it acts to pump carbon dioxide from the atmosphere into the deep ocean. Most of this loss is at high latitudes because the phytoplankton which live there are large enough to sink out of the surface waters into the deep ocean when they die.
|
 |
 |
 |
|
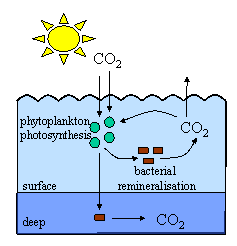
1. This picture gives a very simple idea of how the biological pump operates. Phytoplankton take up carbon dioxide during photosynthesis. Bacteria eat the phytoplankton and, in doing so, release nutrients and carbon dioxide back into the water. This process is known as remineralisation and we will discuss it more in Unit 2 of the Basics Section. Remineralisation mostly happens in the surface waters and the carbon dioxide is either used again by the phytoplankton for photosynthesis or released back to the atmosphere. If the phytoplankton die and sink into the deep waters, the carbon dioxide released by remineralisation is stored for centuries in the deep ocean reducing the impact of global warming. Author: Lucinda Spokes.
|
|
 |
 |
|
2. An electron micrograph of the phytoplankton Emiliania Huxleyi with its calcium carbonate skeleton. Thanks to NOAA for the image.
|
|
 |
Computer models suggest that human activity may alter the types of phytoplankton in the ocean. Humans, therefore, could change how much carbon is stored in the deep ocean. For instance, some phytoplankton produce calcium carbonate skeletons, particularly the very abundant Emiliania Huxleyi. In making their skeletons, these phytoplankton actually cause the release of carbon dioxide and this reduces the overall uptake of atmospheric carbon dioxide by seawater.
|
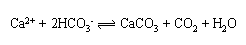 |
 |
|
|
|
At the moment, we don't know all the reasons why certain phytoplankton species grow in particular ocean regions. This means we can't predict whether future human activity will change the abundance of phytoplankton which produce calcium carbonate skeletons and, if so, what impact this will have on our climate.
About this page:
author: Lucinda Spokes - Environmental Sciences, University of East Anglia, Norwich - U.K.
1. sci reviewer: Dr. Marie Jose Messias - Environmental Sciences, University of East Anglia, Norwich - U.K.
2. sci. reviewer: Dr. Holger Brix - Institute of Geophysics and Planetary Physics, University of California, Los Angeles - USA.
edu. reviewer:
last update: 2003-10-22
|
|
 |
|









