|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Basics
!significant changes made! |
Greenhouse gases and effect
The greenhouse effect is very important for life on Earth. If there were no greenhouse gases in the air, the average temperature at the Earth surface would be roughly 30 degrees lower than the 15°C we have now in global average.
|
|
|
|
|
 |
 |
 |
|
1. Greenhouse gases act like a pullover
adapted from: fashion 3sat online
|
|
 |
We see, that we need a natural greenhouse effect like a pullover in winter. However, if the pullover is too thick, we begin to sweat. What man does, is, to make an natural and necessary effect stronger and stronger and therefore an additional warming of the Earth occurs, which has not been the case during the last thousands of years in such an extreme manner. Therefore we are worrying about global warming and the greenhouse effect is often regarded as something bad. But it is not the natural greenhouse effect, which is bad. It is only this additional greenhouse effect caused by humen, which causes all the trouble.
|
|
Greenhouse gases do the same with the heat radiation of the Earth than a pullover does with our body in winter. They hold back the warmth and cause a warm layer around the Earth surface.
|
2. Can you explain the image in your own words? Afterwards you may check and move your mouse over the text, which is written in white between the arrows:
-> The light coming from the sun is mostly visible light. Most of the dangerous UV part is absorbed by the ozone layer. But the visible part goes down to the troposphere. This light may either be reflected by the light parts of the Earth surface (ice, snow) or by the clouds, or it is absorbed by the Earth's surface and heats it up (symbolised by the red colour). <-
author: Elmar Uherek |
Explain also here:
-> 3. Warm infrared radiation (it cannot be seen with our eyes) is emitted by the Earth. The greenhouse gases in the atmosphere (symbolised by blue ellipses) absorb the infrared radiation and send the heat partially back to the Earth, partially to the space. <-
author: Elmar Uherek
|
Which gases contribute to the greenhouse effect?
The most important greenhouse gas is water vapour (making up for about 60% of the greenhouse effect). But it is assumed that the global water vapour content did not change a lot during the last centuries. The concentration of carbon dioxide however, the second important greenhouse gas (contributing with roughly 20%), increased a lot - from 280 to 370 ppm* since preindustrial times. Also methane and ozone show increasing concentrations.
The greenhouse gases are trace gases, accounting (besides from CO2) for 1 Millionth or less of the total air mass.
*1 ppm = 1 molecule among 1 million air molecules
|
 |
 |
 |
|
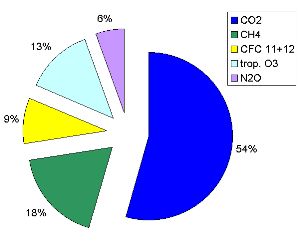
4. Relative contributions in % of each of the tropospheric greenhouse gases to the radiative forcing between 1750 (preindustrial time) and 2000. This is a measure for the additional humen made greenhouse effect. We see that CO2 has the biggest impact.
Diagram: Elmar Uherek, values from IPCC TAR
|
|
|
In some scientific publications the contribution to the Earth's warming is called 'radiative forcing'. It is measured in the unit Watt per square meter W/m2. Since the industrial times began around the year 1750 until now (data from year 2000) the concentrations of greenhouse gases increased a lot due to human activity. The numbers on the right say, how much the 'radiative forcing' increased due to this additional greenhouse gases in the air. The pie chart shows the relative contribution of each of the gases.
|
 |
Radiative forcing of the additional greenhouse gases (1750 - 2000) in W/m2
1.46 CO2 (carbon dioxide)
0.48 CH4 (methane)
0.24 CFC 11+12 (chlorofluorocarbons)
0.35 trop. O3 (tropospheric ozone)
0.15 N2O (nitrous oxide)
Data source: IPCC TAR 2001
|
About this page:
Author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz/Germany
scientific reviewer: Dr. Pascal Guyon - MPI Chemistry - 2004-05-12
educational reviewing: Roland Bergmann + students, Velbert comprehensive school
revised and last published: 2004-05-13
|
|
 |
|









