|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Basics |
Emissions from the Biosphere
There are not only greenhouse gases emitted to the atmosphere, but thousands of other chemical compounds. Many of them are organic compounds and the biosphere (trees, plankton in the ocean and other plants) emits globally seen more of them than humans do.
|
|
|
|
|
 |
|
Carbon is an important element in the living world. Chemical compounds, consisting of carbon, hydrogen, often oxygen and sometimes also a few other elements (nitrogen, phosphor, sulphur) are called organic compounds or organics. Humans emit a lot of them as solvents, in car exhaust and in several chemical industries such as refineries. Since most of us are living in towns or villages it is hard to believe that humans are not the main emittors. But on a global scale there are still wide sparsely populated forest regions and savannahs and certainly the oceans, where natural emissions dominate.
|
 |
 |
|
1. Rice paddy field - Bali, Indonesia
foto by: STRINGER/INDONESIA for Reuters
|
|
 |
What does the biosphere emit?
If you go through a forest or grasslands you get an impression of the smell of numerous organic gases, which are emitted by the trees, the grass or the flowers. World-wide, there are more than 1000 Mio tons emitted every year. Among these compounds huge amounts of the organic species isoprene (about 500 Mio tons per year) and monoterpenes (130 Mio t /yr) are enclosed. In comparison it is estimated that mankind produces about 200 Mio tons of organic compounds per year (methane not included). Smell on the needles of a pine tree, and such a monoterpene will come into your nose.
|
Plants form them and release them through leaves and needles in particular if they react to stress (e.g. heat, drought, injury, ...), but also during their normal life. Also about 200 Mio t / yr of methane have natural sources. However, man contributes too with roughly the same amount from cattle and rice paddies.
We have also to take into account that 71% of the Earth's surface are covered by oceans and there is a lot of living organisms in the water (e.g. algae). Also here, some chemistry is taking place and organic gases are released to the air, e.g. 45 Mio tons per year of Dimethylsulfide (DMS) . This sulphur-organic compound is oxidised to sulphuric acid in the air and leads to the formation of marine clouds.
|
 |
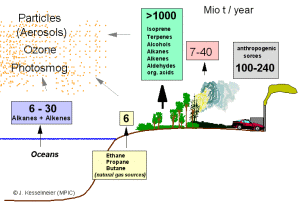 |
 |
|
1.a) Global emissions of gaseous organic compounds (so called VOC) in Mio t per year (Methane and DMS are not included). The compounds are emitted by the oceans, from soil, a major amount from the biosphere (trees and other plants), by fires and from human sources.
Author: Jürgen Kesselmeier
Please click on the image to enlarge it! (30 kB)
|
|
|
Understanding the climate system and the processes in our atmosphere does not only mean to observe, how emissions from man change, but also in which way plants contribute to the global cycles and in which way such contributions are subject to changes. Let's focus on three different examples in order to understand the importance of biogenic emissions.
|
2. The tree as source of organic compounds (following N.C. Hewitt; image: Elmar Uherek)
Isoprene (about 500 Mio t/year world-wide) and monoterpenes (about 130 t/year) are important organic compounds, that are released in high amounts by trees and other plants. But there are many more chemicals plants produce.
Please click on the image in order to enlarge it! (100 kB)
For high resolution (500 kB) -> click here!
|
 |
 |
|
3. The blueish colour of the Great Smooky Mountains (USA) is assumed to come from fine biogenic particles.
|
|
 |
Monoterpenes
Monoterpenes contribute to the smell of many plants, for example the smell of needles in the forest or also the fruity smell of oranges and lemons. Monoterpenes are organic compounds consisting of carbon, hydrogen and sometimes oxygen atoms. They have good sounding names like limonene or pinene. They are produced by trees and other plants, and released most actively with the first sunshine in the morning of warm days. If plants are stressed, for example due to heat or drought or violations, this may enhance the production.
|
|
|
 |
4. On the left you see the chemical structure of a monoterpene, beta-pinene (left), and of one of the most important natural organic compounds, isoprene (right). Both compounds are unsaturated. This means, they have C=C double bonds, highlighted by a red loop. In order to simplify complicated organic molecules, chemists ususally do not draw the C and H atoms. Isopren is shown in both forms, without C and H atoms above and with C and H atoms below.
|
Plant emissions react in the atmosphere
Once emitted to the atmosphere such terpenes react with oxidants (for example OH or ozone). The products are other compounds, which can condense in the air and form particles or make grow already existing particles. These particles, also called aerosols, are floating in the air and they are necessary in order to form clouds. Because different chemicals in aerosols lead to different processes in cloud formation, plant emissions as well as industrial emissions can have a strong influence on cloud formation. Aerosol formation can even be visible: Sometimes you can see it as blue haze over forests (picture 3) and we simulated it with pine needles in the laboratory (picture 5).
|
 |
 |
 |
|
5. Simulation of the blue haze formation in the laboratory (carried out at MPI Mainz).
The beam of a strong lamp helps to visualise the smoke formed in the box, when ozone comes into touch with monoterpenes from pine needles.
Please click to enlarge (60 K)
|
|
 |
 |
|
6. Medicago varia (Fabaceae)
used in agronomy to assimilate nitrogen from the air
Photo: Patrick Knopf, spez. Botanik, Ruhr-Universität Bochum
|
|
 |
Nitrous oxide N2O
Nitrogen is an important element in the living world. It is part of bio-molecules as proteins, amino acids, the DNA or energy carrier molecules which play key roles in every organism. Plants take up nitrogen from nitrate or ammonia in the ground, and bacteria help to make it accessible in this form via nitrogen fixation from the air. But bacteria also decompose nitrate and form in this way nitrous oxide, which is a gas and released to the air. Since nitrous oxide is extremely stable and not destroyed on its way through the troposphere it becomes the most important nitrogen oxide source of the stratosphere. There, it is involved in the reactions which deplete the ozone hole and finally comes back to the ground as nitric acid. The emissions of nitrous oxides have been increased due to increasing fertilisation in agriculture. Roughly 15 Mio t are emitted every year worldwide.
|
Dimethyle sulfide
Little invisible particles of sulphuric acid and water cause cloud formation over the oceans. But where does the sulphuric acid come from? Sulphur compounds are very important especially in the metabolism of the marine biosphere, since sulphate is available everywhere in the oceans. Algae need special sulphur compounds e.g. for the regulation of their water pressure and degradation of them leads to the sulphur organic compound dimethyl sulfide, a gas, which is emitted to the air. There it is oxidised forming sulphur dioxide and finally sulphuric acid, which is necessary for cloud formation.
Unit 3 in the basics sections of the topic 'Oceans' tells you more details about the important role of phytoplancton in the sea.
|
 |
 |
 |
|
7. Visualisation of chlorophyll from phytoplancton.
The satellite image shows the Atlantic ocean east of Canada, but there is not only water in the sea. The animation switches to a visualisaton of the phytoplancton in the sea (increasing concentrations from blue to red), which emits large amounts of dimethyl sulfide to the air.
Source: SEAWIFS Project
|
|
|
The few examples give an idea in which complex and close manner emissions from the biosphere are intertwined with climate processes.
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz/Germany
educational proofreading: Michael Seesing - Uni Duisburg - 2003-07-02
1. scientific reviewing: Prof. Jürgen Kesselmeier - Max Planck Institute for Chemistry, Mainz - 2003-07-15
2. scientific reviewng: Dr. Pascal Guyon - Max Planck Institute for Chemistry, Mainz - 2004-05-10
last published: 2004-06-15
|
|
 |
|









