|
|
 |
|
|
|
 |
| |
|
|
 |
Climate in cities
Basics |
The impact of acid rain on the natural environment
Acid rain affects all elements of the environment e.g. surface waters and groundwaters, soils, vegetation. It hinders food chains and endangers biodiversity. It deteriorates our world.
|
|
|
|
|
 |
 |
 |
|
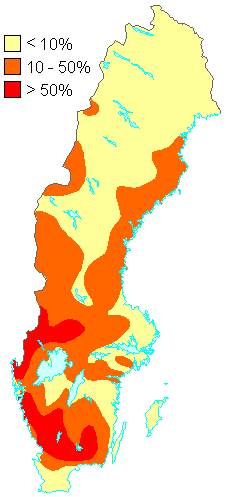
1. Percentage of the total number of lakes in different parts of Sweden judged to be acidified in 1990. The acidification situation has changed little since then.
Source: Swedish Environmental Protection Agency
http://www.internat.environ.se/index.php3?main=/documents/pollutants/kalka/forsure.html
|
|
 |
Acidification of waters
In Scandinavia, acid rain enhances the natural acidity of the lakes and rivers. Some 14,000 Swedish lakes, located in acidic crystalline rocks, are affected by acidification, with widespread damage to plant and animal life as a consequence. The damage also occurs in the United Kingdom and in the Alps. Another region highly sensitive to water acidification is North America.
The decrease in European emissions of SO2 and NOx in the 1990s resulted in recovery for some waters (i.e. the return to pre-industrial levels of acidity and other components that counteract acidity). Great Britain was the only region that did not show a consistent decrease in sulfate concentrations in lakes, although the emissions have been reduced significantly. Reduced emissions, however, do not automatically translate to immediate improvement in streams, lakes and rivers.
|
|
Soils
When soil becomes acidified, its essential nutrients (e.g. calcium (Ca) and magnesium (Mg)) are leached out before trees and other plants can use them to grow, which reduces the soil's fertility. Additionally, aluminum (Al), which in some circumstance may become very toxic and dangerous metal, is released and accumulated in the soil which causes its degradation. Aluminum damages the root hairs and reduces the uptake of phosphorus and other nutrients. The tree later dies from starvation and a reduced resistance.
|
The most endangered soils are those developed on acid rocks, e.g. on quartz sandstone. Soils that are rich in lime are more resistant to acidity. Soils are less vulnerable to acid rain than surface waters, because they have a buffering capacity. This means that the soil may neutralize some or all of the acidity of the acid rainwater. Unfortunately, the buffering capacity can be depleted by continuous deposition of acid rain. In Sweden, in most of the country, the soils are composed of slow-weathering minerals from Scandinavia's Precambrian bedrock, which means that the critical acid load (the maximum acid supply which the soil is capable of neutralizing) is low. Sweden, in other words, is more sensitive to acid deposition than most other countries.
|
 |
 |
 |
|
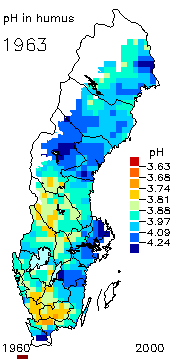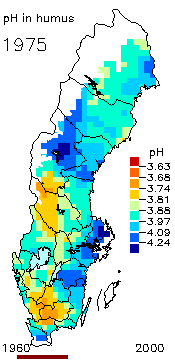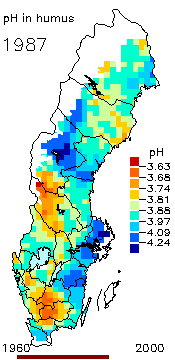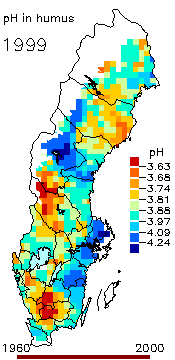
2. The pH level in the humus layer in Sweden. The animation shows its changes since 1963.
Author: Ake Nilsson, Swedish University of Agricultural Sciences.
Source: Swedish Environmental Protection Agency
http://www.internat.environ.se/index.php3?main=/documents/pollutants/kalka/forsure.html
|
|
|
Vegetation
Acid precipitation usually does not kill trees directly; it weakens trees by nutrient loss from the leaves, limiting the nutrients available to them from the soil, or exposing them to toxic substances slowly released from the soil. Acidic pollutant gases, like sulfur dioxide, can cause direct harm to plants growing close to large emission sources such as power stations.
|
 |
 |
|
3. Acidity may cause severe damage to trees. Dead forests in the West Karkonosze Range (the Sudety Mts.), at he Polish-Czech border
Author: Witold Goraczko
|
|
 |
Acidity destroys the surface of the trees’ leaves and needles which causes uncontrolled water loss and hinders photosynthesis. It causes a reduced rate of decomposition of leaf litter and the death of useful microorganisms that are symbiotic with tree roots. Soil organisms (including bacteria) have their respiration rates reduced.
|
Forest damaged by acid rain can be found all over Europe and in many regions of eastern USA. Figures 3 and 4 present damaged forests in the Black Triangle (see link at the bottom of the page).
|
 |
 |
 |
|
4. Mountain forests are exposed to acid clouds, fogs and rain, as well as acidic gases like sulfur dioxide. Dead forests in the West Karkonosze Range (the Sudety Mts.)
Author: Witold Goraczko
|
|
|
In the case of forest damage, the contribution of acid rain is hard to isolate from other stresses such as drought, fire, and pests that figure heavily in forest health. For this reason, the contribution of air pollution to forest damage is a controversial subject, particularly in North America.
|
|
Food chains and biodiversity
Soil acidification releases metals that can harm the micro-organisms in the soil as well as birds and mammals higher up the food chain, including man. The most sensitive groups include fish, lichens, mosses, certain fungi and small aquatic organisms. Acid rain disturbs the natural cycles of sulfur and nitrogen. Some organisms may be completely eliminated (which means a decrease in biodiversity). Acid rain permanently worsens living conditions in an ecosystem. The heavy acidification of soil in the south of Sweden has already brought about substantial changes in biodiversity.
|
 |
 |
|
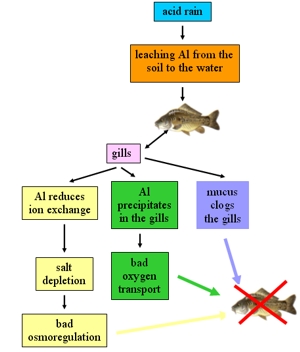
5. Influence of acid rain on fish. See text for explanations!
Author: Anita Bokwa
|
|
 |
Fish and other water-living organisms
When the pH of water is below 5.5, fish die or become seriously ill. Back in the 1950s it was discovered that fish were disappearing from lakes and waterways in southern Scandinavia.
The high level of aluminum (Al) that is leached from the soil by acid rain is the cause of fish death. Firstly, Al is able to reduce ion exchange through the gills and causes salt depletion. For freshwater fish, maintaining osmoregulation (the ability to maintain a state of balance between salt and minerals in the organism's tissue) is essential to stay alive. Aluminum also precipitates in the gills and interferes with the transport of oxygen, so that fish literally die of suffocation. Secondly, fish will exude mucus to combat the aluminum in their gills. This mucus builds up and clogs the gills so that oxygen and salt transport is inhibited. Fish are then unable to regulate their body salts. Also, a low pH level will throw off the balance of salt in the fish's tissue.
Low pH often stunts the growth of frogs, toads and salamanders. A decline in benthos (bottom-dwelling organisms) can lead to a decline in the number of species of flies, mosquitoes, craneflies, midges and mayflies. This puts a stress on aquatic carnivores (such as insect-eating fish). Predatory birds can eat the fish and end up with high concentrations of aluminum. The birds will then produce eggs with soft shells and the young rarely survive. |
About this page:
author: Anita Bokwa - Jagiellonian University, Cracow, Poland
scientific reviewer: Dr. J. Neil Cape - Institute of Terrestrial Ecology, Edinburgh Research Station, Scotland - 2004-08-11
educational reviewing: Michael Seesing - University of Duisburg, Duisburg, Germany
last update: 2004-12-17
|
|
 |
|









