|
|
 |
|
|
|
 |
 |
|
|
 |
Monitoring the fever chart of atmosperic carbon dioxide
|
 |
 |
|
1. 1. Mace Head - Atmospheric watch at the Irish ocean shore - Photo by Tim Lueker, SIO
|
 Monitoring at Mace Head Monitoring at Mace Head
On the west coast of Ireland, at the north end of Galway Bay, you will find at a lonely place in the landscape a cottage and some masts around. It could be the home of a shepherd, but is in fact packed with instruments for atmospheric research. Here, carbon dioxide is measured continuously and additionally samples are taken weekly in 2L or 2.5L glass flasks for external analysis.
|
 |
 |
 |
|
2. The research station at Mace Head: Here the University of Galway, an ACCENT partner, and several international teams carry out continuous atmospheric monotoring as well as temporary field campaigns.
|
|
 |
 |
|
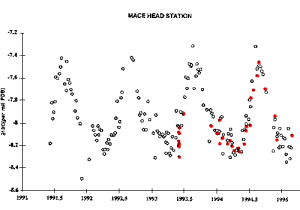
3. Comparison of CO2 values, sampled at Mace Head and independently analysed in Boulder (open circles) and in France (red filled circles).
|
|
 |
The concentration of carbon dioxide is determined by analysis in the NOAA Climate Monitoring and Diagnostics Laboratory (CMDL) in Boulder, Colorado (USA) by gas chromatography. This reference laboratory compares the results with other laboratories worldwide. Analysis of flasks from Mace Head is also carried out in a laboratory in France using mass spectrometry. The Mace Head station is only one in the network of Global Atmosphere Watch, which compares atmospheric data world wide: mixing ratios of carbon dioxide, carbon monoxide, methane, sulphur dioxide, dinitrogen oxide and others.
|
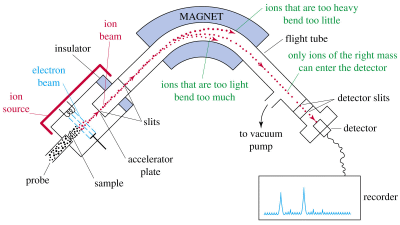
4. A traditional mass spectrometer
Mass spectrometry is a method of analysing molecules of different molecular masses. The molecules are positively charged by electron bombardement, which takes away one electron. The charged molecules (ions) go through a bent electric field and depending on their mass their path is either bent just in the right manner in order to reach a dectector or they end up on the walls of the field block. Different masses can be separated and detected by changing the strength of the field.
|
 |
 |
 |
|
Mass spectrometer, source: University of Wisconsin
|
|
 |
 |
|
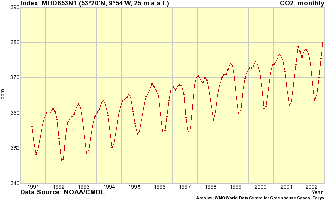
5. Monthly CO2 mixing ratios from Mace Head © Global Atmosphere Watch
|
|
 |
 What has an influence on CO2 concentrations? What has an influence on CO2 concentrations?
Basically, carbon dioxide is homogeneously spreading world-wide and the mixing ratios at two different places vary only by a few parts per million (ppm). Very accurate data however depend also on the local sources and sinks of CO2. For example large towns and coal power plants emit a lot of CO2. The Mace Head station is close to the ocean. Here phytoplankton (algae) take up carbon dioxide and partially transport it to the deeper ocean layers when dying. In the growing season also the big forests are sinks of carbon dioxide and convert it into biomass.
|
 |
 |
|
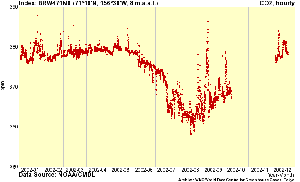
6. Hourly CO2 mixing ratios for the year 2002 measured at Barrow (Alaska). © NOAA, Global Atmosphere Watch
Click on the graphs for a larger view!
|
|
 |
Taking into account all these factors, we will find a seasonal variation of CO2 but also some peaks from temporary disturbance as you can see in the high resolution diagram from Barrow on the left.
Gerard Spain is research assistant in Mace Head and answers our question: How can we make sure that only clean air, hardly influenced by human emissions (so called 'background' air), is measured in the weekly or monthly glass flasks?
|
"The flask samples are normally only collected under clean, background conditions. When non-background air is sampled, it is either done so as to collect a sample representative of continental air (coming for example from the industrial regions in Europe) or simply to avoid a large gap in the data record. In either case it is noted as such and is not included in any "background" analysis.
To avoid accidental sampling of polluted air, weather forecasts and forecast trajectories (predictions of where the air will come from) are used to select suitable days for sampling. In addition, we measure many trace gas species and aerosol parameters in real- or near-realtime. These can be used as a diagnostic to determine how "clean" an air mass is. Finally, back trajectories (computer calculations of where an air mass has come from) are used to identify the source region assoociated with each sample and these are also a confirmation that the sampled air is background."
|
 |
 |
 |
|
7. Gerard Spain is research assistant at Mace Head
|
|
|
 Problems to solve in analytical science Problems to solve in analytical science
What do scientists have to think about when measuring CO2 mixing ratios? Some rules of good analysis are also important for many of your classroom results.
|
Sampling:
Most mistakes are made during sampling. Samples should not be taken directly in an exhaust gas plume. Imagine you measure CO2 after a lesson in your classroom. What result do you expect compared to a probe taken on the free field?
Consider if the sampling place is representative for the region and if the result is also valid in other places. If a compound has strong seasonal or even daily variance, one probe per year or per month cannot be enough.
|
 |
 |
 |
|
8. Measuring carbon dioxide uptake rates of trees (here by a branch cuvette in Hyytiälä / Finland) is much more uncertain than measuring the relatively stable CO2 mixing ratios in the air.
Click the photo for a larger view! (160 K)
Source: University of Helsinki
|
|
|
Contamination:
Is the sampling vessel clean and free from older air? Vessels should be evacuated and flushed. Can compounds stick to the wall of a sampling vessel if the sample is stored for longer time? The air should not come into touch with the air in the laboratory where it is measured.
|
 |
 |
|
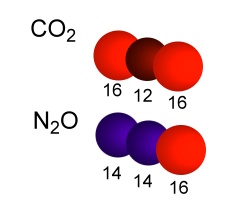
9. Calculate the masses of CO2 and N2O molecules. What is the problem in mass separation in a simple mass spectrometer?
image: Elmar Uherek
|
|
 |
Reference:
Carbon dioxide is compared against a reference. The composition of the reference may change over time. It has to be well prepared.
Instrument:
Is the instrument sufficiently exact? Can CO2 and N2O (both are stable greenhouse gases) be distinguished in parallel only by molecule mass with a simple mass spectrometer?
|
|
Scientists often spend a lot of their research time trying to improve the techniques and to avoid the potential mistakes described above. Nowadays, excellent instruments are available but mistakes can easily be made in sampling, owing to contamination or reference problems.
Author: Elmar Uherek
Max Planck Institute for Chemistry, Mainz, Germany
|
|
 |
|







