|
|
 |
|
|
|
 |
 |
|
|
 |
Fundamentals B1: Phase transitions
 How can a gas be understood? How can a gas be understood?
The gaseous state seems not very tangible to us. But also gases are gases and not liquid or solid matter only because there is enough energy (heat) in their environment so that their molecules do not stick to each other and do not aggregate.
|
Imagine a room with a vibrating floor and many balls on this floor. The balls are continuously shot upward by the vibration so that the whole room is full of flying balls. This is roughly the state in a gas. It is a state of constant mobility of the gas molecules, which is due to the available heat energy. We call this Brownian motion of the molecules.
|
 |
 |
 |
|
1. Screen copy - Illustration of the Brownian motion
|
|
|
But this means also, that at very low energies in the environment (when it is very cold) gaseous compounds can become liquid or solid. For example carbon dioxide can be bought in blocks as dry ice. It becomes solid at about -78°C. Even nitrogen, one of the lightest gases in the air, can be kept in a liquid state at very low temperatures. Nitrogen becomes liquid at -196°C.
|
 |
 |
|
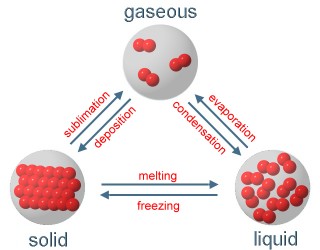
4. Phase transition between the three physical conditions; Scheme: Elmar Uherek
|
|
 |
We see: The substances of the air can become liquid or solid at very low temperatures, but they are gaseous at temperatures between -60°C and +50°C which are common on our planet Earth.
If a gas becomes liquid we call it condensation, if it becomes immediately solid we call it deposition.
|
|
 Phase transition for water Phase transition for water
It is not hard to imagine that there are compounds, which turn more easily into a liquid or solid state than nitrogen or carbon dioxide. The best example is water.
|
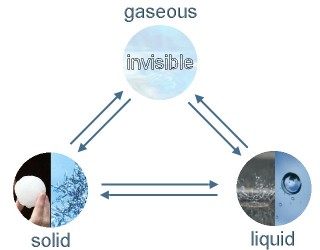
Water evaporates and changes to water vapour in the air. It becomes rather visible after a rain shower in summer when the warm streets are really steaming. Water vapour can condense in the air and cloud droplets are forming. If they become too big they fall to the ground as rain. High clouds in very cold air levels can consist of ice crystals. When hail or snow is falling solid water even reaches the ground before it has been melting.
|
 |
 |
 |
|
5. Phase transitions and physical states of water: ice / hail / snow, liquid water, water vapour
Image: Elmar Uherek
|
|
|
 |
|







