|
|
 |
|
|
|
 |
 |
|
|
 |
Research B: Formation of Aerosol - Role of sulfuric acid
 Amount of particles in air Amount of particles in air
We described in the research text part A how fine particles of a diameter of less than 10 µm are measured with a common particle analyser for ambient air control.
|
|
With the help of such analysers we can get an overview which amounts of particles are found in rural regions, in the environment of a city and directly in the city centre.
|
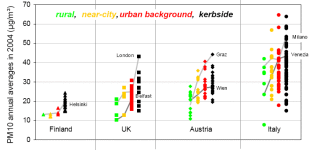
In average concentrations measured in the city centre (urban background) and immediately next to the street ( kerbside) are higher than concentrations in rural regions or in the wider environment around a city. However, the differences between a city and its environment are often less sharp if the whole region is strongly polluted.
|
 |
 |
 |
|
1. PM 10 annual average in different states in Europe. Grey lines connect rural, near-city, urban background and kerbside sites that belong to the same urban area. Source: Jean-Philippe Putaud, JRC, and EEA/AIRBASE.
Please click to enlarge!
|
|
|
 Sulfuric acid and particle formation Sulfuric acid and particle formation
But also in remote uninhabitated regions there are always certain amounts of fine particles in the air. We assume two major sources for these particles: sulfuric acid and low volatile organic compounds in the air. On this page we discuss only sulfuric acid.
|
 |
 |
|
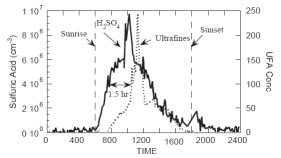
2. Time series of sulfuric acid (black line) with subsequent formation of new particles (dotted) at Idaho Hill, Colorado; 1993-09-21 (Weber et al., 1997).
|
|
 |
The formation of sufate compounds from sulfuric acid in the air leads to very fine particles. During their formation their size is smaller than the detection limits of particle analysers. It has been observed during a field campaign in the Rocky Mountains that the formation of finest particles took place after a strong increase in the concentration of sulfuric acid (H2SO4) in the atmosphere.* This increase occured from morning to noon.
|
|
Particle formation can take place if a certain saturation with sulfuric acid in the air has been reached and if a certain humidity is given at the same time. The critical point for new particle formation is reached much earlier, if traces of ammonia (e.g. 1 ppt = part per trillion) are in the air. In this case sulfuric acid and ammonia can form ammonium sulfate, a very hygroscopic salt. Since water as the third partner plays still a role, we call this formation of new particles "ternary nucleation" (nucleation with three partners).
 What do we observe if new particles are formed? What do we observe if new particles are formed?
In the following series of images we try to illustrate what is observed by a scientist during the formation of new particles and what is really taking place.
|
 |
 |
|
But we cannot be sure about this. It can also be that the sulfuric acid just sticked on the pre-exisiting clusters and maybe even mixed with other compounds in the particle.
|
|
 |
Consequently, the moment of birth of a particle is still rather in the dark for us. Many very small particles which have been just forming stick to larger ones before they reach a size which is detectable for our instruments. The smaller the growth rates of particles are and the more particles have already been in the air, the more unlikely does it become to observe ultra-fine particles in the range between 3 and 20 nm. But since we can well generate ultra-fine particles in the laboratory from water and sulfuric acid we can assume that they form also in the atmosphere.
|
|
 Chemistry of sulfuric acid formation Chemistry of sulfuric acid formation
We see in figure 2 that sulfuric acid is formed at a certain time of the day. This coherency is not surprising. Sulfuric acid is formed by oxidation of sulfur dioxide. The necessary oxygen compounds are primarily formed in chemical reactions, which are driven by the solar energy during the day. Sulfur dioxide has natural sources (emissions from algae and volcanoes) and since the industrialisation it is also released in larger amounts to the atmosphere by humans (combustion of coal and heavy oil as well as industrial processes).
|
4. In the atmosphere, sulfuric acid is formed on different rather complex ways, in the gas phase as well as in the liquid phase. The scheme on the right shows two important ways: the oxidation with hydroxy-radicals in the gas phase and the oxidation with ozone (or other soluble oxidants) in the liquid phase, i.e. in cloud or fog droplets. Also the reaction of sulfur trioxide with water is not a simple addition. Several water molecules are involved.
Scheme: Elmar Uherek; Please click to enlarge!
|
 |
|
* It is a problem for aerosol researchers that ultra-fine particles are indeed detectable but their chemical composition cannot be determined, since the amounts are too small. Therefore, the theory of new particle formation from sulfuric acid is conclusive but not finally proven.
|
|
 |
|







