For salts it is even easier than for water to turn into a solid state. Their smallest particles, the positive and negative ions, attract each other heavily.
Just ask your parents for an old pot, which is not needed anymore. Put it on the stove and give a teaspoon of dry table salt into it. Switch the stove on and choose the highest grade. What happens?
|
 |
 |
 |
|
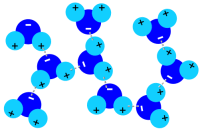
1. Polar hydrogen bonds in liquid water; image: Elmar Uherek
|
|







