|
|
 |
|
|
|
 |
| |
|
|
 |
Higher Atmosphere
Basics |
Formation of ozone
The biosphere, the animals on our planet and certainly also human life became possible due to the formation of ozone in the stratosphere. Ozone protects us from the UV-C and UV-B radiation of the sun (light with wavelength of less than 320 nm), which damages the biomolecules. In Earth's history ozone could form after the release of oxygen to the atmosphere between 2000 and 600 Mio before our time. But how?
|
|
|
|
|
 |
 |
 |
|
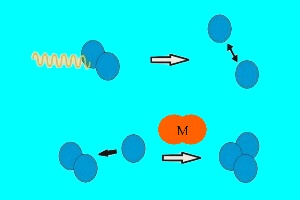
1. ozone photosynthesis - light splitting oxygen molecules leads to ozone formation
|
|
 |
UV light drives ozone formation and destruction
In the stratosphere there are two forms of oxygen: normal oxygen O2 consisting of two O atoms and ozone O3 consisting of three O atoms. In order to transform one form into the other intensive UV-light is necessary. The O-O bond of an oxygen molecule is broken. The formed O atom reacts with oxygen (and for energetic reasons a collision partner M) and forms ozone. This UV-light (yellow wave in the image on the left) comes from the sun.
|
 |
 |
|
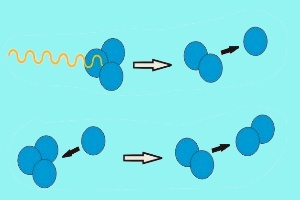
2. ozone photolysis - degradation by sunlight
|
|
 |
In an analogous way, ozone is destroyed by photolysis, if the O-O bond in an ozone molecules is split by sunlight. In this case the formed O atom reacts with another ozone molecule and forms two oxygen molecules O2.
Formation in the tropics, accumulation in polar regions
Since solar radiation is strongest over the tropics, here most of the global ozone is formed. However, the sun in the tropics does not only drive ozone formation, but also the rise of tropospheric air to high altitudes. |
Ozone is transported away from the equator towards the poles, where it accumulates in the cold subpolar regions. At the equator itself, ozone formation as well as photochemical depletion are high. Ozone cannot accumulate. In the subpolar regions however, photochemical depletion is low and the transport from equator is an important source. The values at the poles themselves are lower, in particular in winter, when no additional ozone can be formed during the polar night. Therefore the highest ozone values are observed over the polar regions, as long as the system is not disturbed by the ozone hole formation in Antarctic spring.
3. The scheme on the right shows the ozone transport in theory. The included simulation shows the measured ozone distribution between the pole and the equator (low values = blue, high values = red). It is obvious that near the equator air of low ozone content raises to high altitudes. Data from GOME (DLR, IUP Bremen).
Please click to enlarge (100 K)!
|
 |
|
 |
 |
|
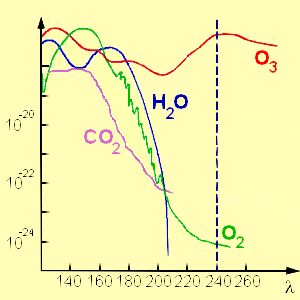
4. Absorption of ozone compared to other atmospheric compounds absorbing in the UV range of the sunlight.
Source: adapted from Chemie Didaktik Uni Duisburg
|
|
 |
Ozone absorbs UV light
The same UV light which is necessary for the ozone formation is also absorbed by the molecules of the ozone layer. This has three consequences:
1) This light does not reach the lower parts of the atmosphere and therefore the Earth surface is protected.
2) The amount of ozone, which can accumulate, is limited. With increasing ozone concentrations the probability of a decomposition increases too. This leads to an equilibrium.
3) UV light contains energy. The energy is transformed into heat radiation and leads to a warming of the stratosphere. This is the reason for the inversion of the temperature trend at the tropopause.
|
Thickness of the ozone layer
The term ozone layer is often misunderstood. Ozone layer means, that compared to the troposphere below and the mesosphere above a higher fraction of ozone molecules is found in the stratosphere between 18 and 40 km of altitude. However this fraction is certainly very small compared to nitrogen and oxygen. Not more than 10 of 1 million molecules are ozone. But since ozone in the troposphere is even less and 90% of all atmospheric ozone is in the stratosphere, we call this region the ozone layer. This does not mean that there is somewhere in the atmosphere a thin film of ozone.
|
Dobson units (DU)
However, ozone is often measured in Dobson Units (DU). 300 DU is a typical value. What does it mean? If we assume that all ozone molecules would not be spread over the whole stratosphere and 10% also over the troposphere, but concentrated in a small film at the ground, the thickness of this film would be about 3 mm (= 300 DU). 1 DU = 0.01 mm film thickness of pure ozone at the ground.
|
 |
|
About this page:
author: Dr. Elmar Uherek - Max Planck Institute for Chemistry, Mainz
educational proofreading: Michael Seesing - Univ. of Duisburg - 2003-07-02, Dr. Ellen K. Henriksen - Univ. of Oslo
scientific reviewer: Dr. John Crowley - Max Planck Institute for Chemistry, Mainz 2004-05-06
educational reviewing: Hendrik Förster & students, Nordpfalz Gymnasium Kirchheim-Bolanden - March 2004
last published: 2004-05-10
|
|
 |
|









