|
|
 |
|
These experiments will give you an impression, what the function of the greenhouse gases carbon dioxide and water is.
The absorbtion of heat radiation will be investigated. |
 |
 |
|
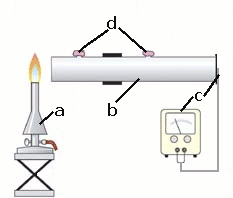
Figure 1
experimental set-up for experiment 1
a: gas burner; b: metal tube (made from cans) with two openings; c: temperature sensor with indicating instrument; d: play dough to close openings;
© 2004 Tausch, Seesing; Uni-Duisburg-Essen, Duisburg [Lit.: Tausch, von Wachtendonk: Chemie 2000+; Buchner Verlag; Bamberg 2001]
|
|
 |
Heat absorption:
Construct the set-up according to Fig.1. In order to make the metal tube, remove the lids and bases from two cans. Connect the cans airtight with tape and drill two holes into the tube as openings. Close these openings with play dough. Close one end of the metal tube with an airtight lid made of black cardboard that is fixed with scotch tape. Attach the tip of the temperature sensor to the cardboard using scotch tape as well. The back is covered with aluminium foil. This way you get a detector of heat radiation. The heat radiation warms the black cardboard which can be seen at the measuring device as a rise in temperature. Make sure you place the burner at the same distance (10 to 15 cm) from the other end (open or covered by different materials: see E1 or E2) of the metal tube during all of your experiments! During each series of measurements read the temperature every 30 seconds, take it down and plot it graphically. Carry out each series of measurements for 3 minutes. Before you start your next series of measurements cool down the metal tube to the initial temperature (you might need a cold air hair-dryer). |
|
E 1 |
Carry out the series of measurements with the following materials covering (e.g. with an elastic band) the big opening of the metal tube. |
|
gas inside the tube: air |
temperature [°C] after: |
|
material: |
0 s |
30 s |
60 s |
90 s |
120 s |
150 s |
180 s |
|
a) polyethene-foil |
|
|
|
|
|
|
|
|
b) aluminium- foil |
|
|
|
|
|
|
|
|
c) flat bag made of polyethene (empty) |
|
|
|
|
|
|
|
|
d) flat bag made of polyethene
(moistened with water inside) |
|
|
|
|
|
|
|
|
e) nothing (open end of metal tube) |
|
|
|
|
|
|
|
|
E2 |
Repeat your series of measurements a) from E1 (air inside the tube) but fill the tube with carbon dioxide this time and close the small openings with play dough again. |
|
gas inside the tube: carbon dioxide |
temperature [°C] after: |
|
material: |
0 s |
30 s |
60 s |
90 s |
120 s |
150 s |
180 s |
|
f) polyethene-foil |
|
|
|
|
|
|
|
|
T 1 |
Why do you measure with the set-up from Figure 1 the propagation of heat by heat radiation rather than the convection of gas quantities? Give reasons for your answer. | |
|
|
T 2 |
Why is it necessary after each series of measurements to cool the temperature down to the initial temperature of the first series of measurements? |
|
T 3 |
The graphic depiction of the experimental results with air inside the metal tube leads to temperature-time-curves. After 3 minutes the temperature has risen highest in series e) with no cover at the big opening.
The following order can be observed:
e) > a) » c) > d) » f) > b)
Interpret these results regarding the absorption of heat radiation through the tested materials and arrange the materials in an order of decreasing heat absorption. |
|
T 4 |
Why do you have to repeat measuring series a) (polyethylene foil) with carbon dioxide if you want to learn about heat absorption of carbon dioxide? |
|
T 5 |
Carbon dioxide CO2 is known as greenhouse gas which contributes to global warming. Nevertheless a measuring series with CO2 in the metal tube has shown that the temperature does not rise to the same extent as it does with air inside the metal tube.
What might be the reason for this? Please mark the correct answer(s): |
c This result is wrong, the experiment has to be repeated.
c CO2 is not a greenhouse gas.
c CO2 can absorb heat better than air, therefore the temperature
does not rise as high.
c This experiment has nothing to do with the greenhouse effect.
|
 |
 |
|
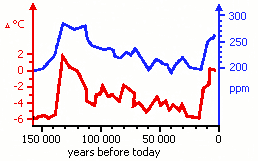
Figure 2
The amount of CO2 in the atmosphere (right axis, blue curve) and relative temperature (left axis, red curve) during the previous 160 000 years.
© 2004 Seesing, Tausch; Universität Duisburg-Essen, Duisburg [Lit:Tausch, von Wachtendonk: 2000+, Buchner Verlag, Bamberg 2001]
|
|
It is possible to measure the amount of CO2 in the atmosphere of ancient times by analysing trapped air in ice cores (Fig. 2).
|
T 6 |
Describe the most important tendencies shown in Figure 2. |
|
T 7 |
Which of the statements from exercise T5 is contrary to the information from Figure 2? Give reasons for your answer. |
|
About this page:
Authors: M. Seesing, M. Tausch - Universität Duisburg-Essen, Duisburg / Germany
Rewiewer:
Last update: 2004-05-13 |
|
 |
|









