|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more |
Worksheet 2
A model experiment on the greenhouse effect
|
|
|
|
|
 |
 |
 |
|
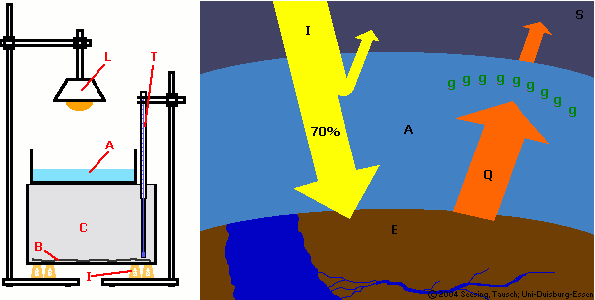
Figure 1: set-up for
experiment 1
L: lamp; T: thermometer
A: water; C: carbon dioxide
B: black cardboard
I: heat insulation (cork plugs)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen |
Figure 2: greenhouse effect:
a great part of the sunlight (I) reaching the earth (E) is absorbed, transformed into heat (Q) and the warmer earth reemits longwaved heat radiation. Greenhouse gases (g) from the atmosphere (A) absorb this reemitted heat almost entirely and send it partially back to earth. The effect is that they keep the heat like in a greenhouse.
(S: space)
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg |
|
|
|
E1 |
Set up the experiment according to Figure 1. The set-up consists of a 300 W (tungsten) lamp, a glass container filled with water (1 cm high), a second glass container with black cardboard covering its ground and a temperature sensor in the gas space of the lower glass container.
After switching on the lamp read the temperature every 30 seconds, take down the values and make a graph. Finish the measuring series after 5 minutes. |
|
T1 |
Carry out measuring series with a) air and b) carbon dioxide in the lower glass container. |
|
T2 |
Repeat measuring series a) and b) after having exchanged the black cardboard with aluminium foil. |
| |
temperature |
|
|
ground: black cardboard (A1) |
ground: aluminium foil (A2) |
|
time [sec] |
air |
carbon dioxide |
air |
carbon dioxide |
|
0 |
|
|
|
|
|
30 |
|
|
|
|
|
60 |
|
|
|
|
|
90 |
|
|
|
|
|
120 |
|
|
|
|
|
150 |
|
|
|
|
|
180 |
|
|
|
|
|
210 |
|
|
|
|
|
240 |
|
|
|
|
|
270 |
|
|
|
|
|
300 |
|
|
|
| |
|
|
T3 |
Write down which of the parts from experiment E1 is a simulation of which part from nature (c.f. Fig. 1 and Fig. 2). |
|
The lamp simulates |
___________________________________________________ |
|
The water in the glass container simulates |
___________________________________________________ |
|
The air / the carbon dioxide from the lower glass container simulates |
___________________________________________________ |
|
The black cardboard on the bottom of the lower glass container simulates |
___________________________________________________ |
|
T4 |
Experiment E1 is a model experiment simulating the greenhouse effect. Why is it important to place cold water between the lamp and the gas space on the lower glass container? Please check the correct answer. |
The function of the layer of water between the lamp and the gas space is:
c It absorbs the heat coming from the lamp.
c It absorbs some parts of colour from the spectrum of the light of the lamp.
c It prevents the gas from leaking out of the container.
c It simulates the humid clouds in the atmosphere.
|
 |
 |
|
Figure 3: Results from two measuring series.
© Tausch, von Wachtendonk: Chemie 2000+;
Buchner Verlag, Bamberg 2001
|
|
 |
|
T5 |
After approximately 150 s the measuring series with CO2 reaches a temperature which is about 1°C higher than the temperature from the measuring series with air in the gas space. Explain this observation using the information from Figure 2: | |
|
|
T6 |
In the model experiment the greenhouse effect becomes especially apparent if there is black cardboard on the bottom of the gas space. You can also get satisfactory results using coloured cardboard, but not using aluminium foil. Find an explanation for this. |
|
T7 |
Due to greenhouse gases the air next to the earth surface is in averange 33°C warmer, than it would be the case without these gases. Of this 20.6°C are caused by water vapour (H2O) from the atmosphere, 7.2 °C by carbon dioxide (CO2), and 2.4 °C by ozone (O3) that is close to the ground. The air close to the ground contains 0.037% vol. parts CO2, but only ca. 40 ppb O3. How many °C would be the contribution of ozone to the greenhouse effect if this contribution was proportional to its amount in the air? Please write down your calculation and result in the space below: |
Compared to CO2 ozone contributes with _____________°C to the greenhouse effect.
|
T8 |
In reality the contribution of ozone to the greenhouse effect is much higher than according to the calculation from T7. What might be the reason for this? |
c Ozone is a stronger oxidising agent than the other greenhouse gases.
c Ozone absorbs heat radiation at wavelengths that are not absorbed
by the other greenhouse gases.
c The amount of oxygen in ozone molecules is much higher than in the
other greenhouse gases.
c The ozone close to the ground is formed under strong incident solar
radiation.
|
About this page:
Authors: M. Seesing, M. Tausch - Universität Duisburg-Essen, Duisburg / Germany
Rewiewer:
Last update: 2004-05-13 |
|
 |
|









