|
|
 |
|
|
|
 |
| |
|
|
 |
Lower Atmosphere
Read more |
Worksheet 2
A model experiment on photosmog
|
|
|
|
|
 |
|
Mention: Some chemicals used in the experiment are harmful. Experiment carefully and protect yourselves!
|
 |
 |
|
Figure 1: Model experiment on photosmog.
©Tausch, von Wachtendonk: Chemie 2000+; Buchner Verlag, Bamberg 2001
|
|
 |
|
E1 |
Irradiation: (demonstration experiment, Work in a fume hood! Use aluminium foil as UV-protection!)
A 450-mL-water-cooled immersion-lamp reactor is filled with 2mL tetrachloroethene [Xn,N; R: 40-51/53; S: 2-23-36/37-61], ca. 1 cm high with glass beads or glass rings and with fresh leaves.
Turn on the water-cooling and irradiate for 25 minutes with a 150 W -UV- immersion-lamp (high-pressure mercury vapour lamp). |
|
E2 |
Extraction, filtration: (group experiment)
Give cut leaves and quartz sand into a mortar and grind it with methanol [T,F; R: 11-23/24/25-39/23/24/25; S: 1/2-7-16-36/37-45]. Filter the green solution afterwards.
Make extracts from irradiated and non-irradiated leaves of the same kind. |
|
E3 |
Thin layer chromatography: (group experiment)
Divide a TLC-aluminium foil coated with silica gel into two sections and add two points of application from the two extracts from E2. For the development of the thin layer chromatogram use as solvent a mixture containing petroleum ether (with a boiling-point range of 30-50°C)[F+,Xn,N; R: 12-51/53-65-66-67; S: 9-16-29-33-61-62], benzine (with a boiling-point range of 100-140°C)[F, Xn, N; R: 11-38-51/53-65-67; S: 9-16-23-24-33-61-62] and 2-propanol [F, Xi; R: 11-36-67; S: 2-7-16-24/25-26] in a ratio of 25:25:5. |
|
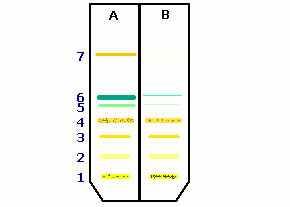
In this model experiment you obtain different results according to the kind of leaves used and the time of irradiation. Nevertheless these results will be very similar to those depicted in Figure 2.
|
T1 |
Which pigments have been hurt or destroyed?
How can you recognise that? |
|
 |
 |
 |
|
Figure 2: Thin layer chromatogram of extracts from non-irradiated (A) and irradiated (B) leaves.
[1: point of application; 2,3,4: xanthophylls; 5: chlorophyll a ; 6: chlorophyll b; 7 ß-carotene]
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg
|
|
|
|
T2 |
The experiment on the damage of leaves (E1) is a model experiment for processes that can also occur in nature. The reaction conditions inside the reactor are not quite the same as in nature though. Partially they are stronly exaggerated concerning processes in the troposphere and in the stratosphere.
Add suitable keywords in the table below (e.g. higher, lower, shorter, longer, different, almost the same, etc.) comparing the processes in the troposphere and stratosphere with the experiment: |
| |
troposphere |
stratosphere |
|
gas pressure
|
|
|
|
composition of the gas mixture |
|
|
|
time of irradiation
|
|
|
|
periodicity of the irradiation (day/night rhythm) |
|
|
|
wavelength of the
light ( l )
|
|
|
|
open / closed system |
|
|
|
dynamics of the gas masses
|
|
|
|
temperature of the gas mixture
|
|
|
|
 |
 |
|
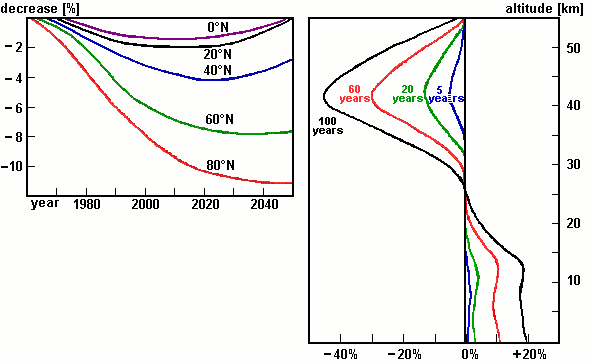
Figure 3: Prognoses on the change of the total ozone column referring to geographic latitude (left) and to the change in the vertical ozone distribution (right). [Lit.: G. Mégie, Ozon, Springer, Berlin 1989]
© 2004 Seesing, Tausch; Universität-Duisburg-Essen, Duisburg
|
|
|
T3 |
Interpret the ozone prognoses from Figure 3. What is more alarming, the change in the total ozone column or the vertical distribution? Give reasons for your answer. |
|
T4 |
Look at the vertical distribution of ozone in Fig. 3 (right) in "60" years. Which phenomenon is represented by the model experiment?
1. ozone hole
2. photosmog
3. ozone hole and photosmog
Give reasons for your answer. |
|
T5 |
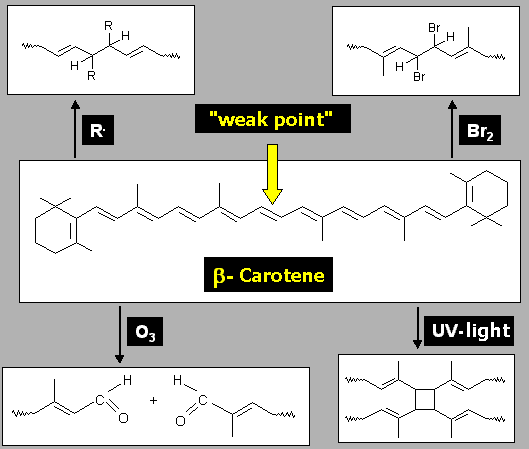
ß-carotene has a weak point that can be attacked very easily.
Which of the possible reactions from Fig. 4 may take place in experiment E1?
Give reasons for your answer. |
|
 |
 |
|
Figure 4: possible reactions of ß-carotene
© 2004 Schmidt, Seesing, Tausch; Universität-Duisburg-Essen; Duisburg
|
About this page:
Authors: M. Seesing, M. Tausch - Universität Duisburg-Essen, Duisburg / Germany
Scientific reviewer: Dr. Rolf Sander - Max Planck Institute for Chemistry, Mainz - 2004-05-18
Last update: 2004-05-13 |
|
 |
|









