 |
 |
|
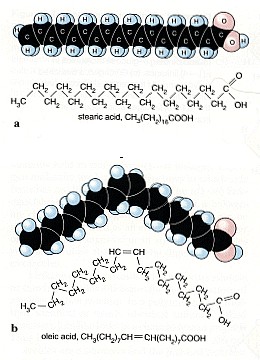
2. Not all fatty acids have double bonds. Some are saturated (a). But many of them are unsaturated, i.e. they have C=C double bond (b)
Source: NTRC, Univ. of Texas Kingsville
|
|
 |
Ozone attacks our respiratory system
After our first year in organic chemistry we know: Carbon C and hydrogen H are present everywhere in living organisms, in plants, in animals and also in our body. Besides from C and H there may also be oxygen O, nitrogen N, sulphur S and phosphor P in organic compounds, but hydrocarbons form the backbone. This backbone includes C-C single bonds, which are very strong and C=C double bonds, which are in some way easier to attack. For ozone in particular the double bonds are interesting. We find them everywhere: in unsaturated fatty acids, haemoglobin, proteins and many other bio-molecules, also on the surface of the pulmonary alveoli in our lungs and its mucous membrane.
The other two main oxidants, OH and NO3 have an extremely short lifetime and react immediately when they are formed. Ozone however finds its way down to the lungs. Every day 20,000 liters of air pass the tiny alveoli, which have a total surface of 80-100 square-meters. Ozone can penetrate and react.
|









